ORGANIC ACIDS
Besides fulvic and humic acids, MINERAL-FUL ( MLG-50™) products are rich in these 13 organic acids and an additional 18 amino acids.
- Certified OMRI Listed
- Highest FA content available
- with documented analysis
- Fulvic content: 15%-20%*
- Trace minerals and electrolyte content: 70
- Compliant for all organic applications
- Available in liquid
- Safe for human consumption
The benefits of 13 organic acids in MINERAL-Ful (MLG-50™)
GALLIC
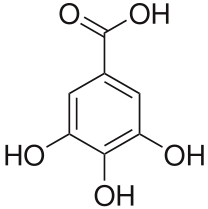
Gallic acid concentrations alleviated toxicity in rice by assisting in the increased level of antioxidant activity and photosynthetic efficiency (Ozfidan-Konakci, Yildiztugay, & Kucukoduk, 2015, p. 219).
Gallic acid mitigated toxin-induced injury, having a beneficial effect on salinity and osmotic stress (Ozfidan-Konakci, Yildiztugay, & Kucukoduk, 2015, p. 1487).
A gallic acid derivative has potent growth promoting action on plants (Bhattacharya, U.S. Patent No. WO 2005063677 A1, 2005).
Gallic acid, an auxin synergist (auxin synergists have been long proven to promote cutting root growth), promoted cutting root growth in the plant Eranthemum tricolor (Randhawa & Mukhopadhyay, 1986, p. 51).
Gallic acid is known to have a pronounced effect on insect or pathogen infections in plants, acting as a defense against such herbivores (Ananthakrishnan, 1997, pp. 577-578).
CAFFEIC
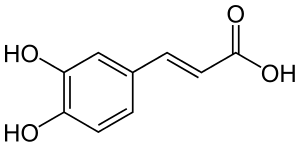
Applying caffeic acid to plants increased the N fixation rate, seed weight and other plant productivity measures (Neera & Garg, 1989).
Caffeic acid increased soil respiration and microbial biomass in fallow or almost-fallow soils (Sparling, Ord, & Vaughan, 1981, p. 455).
When applied to soybean shoots, caffeic acid relieves the following effects of saline soil: the results of growth-inhibiting stress on the shoots, chlorophyll loss and inhibition of nitrogen fixation (which is essential to plant growth) (Klein, Keyster, & Ludidi, 2015, p. 13).
Caffeic acid may enhance nitric acid biosynthesis (Klein, Keyster, & Ludidi, 2015, p. 13).
FUMARIC
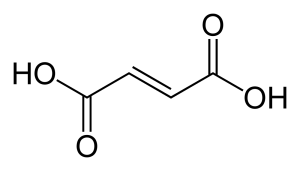
Rockcress leaves showed an increase in fumaric acid as the plant aged and was exposed to increasing light intensity (Chia, Yoder, Reiter, & Gibson, 2000, p. 743).
Fumaric acid can be metabolized by the plant for energy (Chia, Yoder, Reiter, & Gibson, 2000, p. 743).
Fumaric acid is mostly found in photosynthetically active tissues. Studies suggest that an increase in photosynthetic activity leads to an increase in the accumulation of fumaric acid in photosynthetic tissues (Chia, Yoder, Reiter, & Gibson, 2000, pp. 746 & 748).
Fumaric acid is synthesized in plant mitochondria (the energy production powerhouses) and may function as a source of energy for the plant as well (Chia, Yoder, Reiter, & Gibson, 2000, p. 749).
Fumaric acid can be a major form of fixed carbon (which contributes to plant growth) (Chia, Yoder, Reiter, & Gibson, 2000, p. 750).
SHIKIMIC
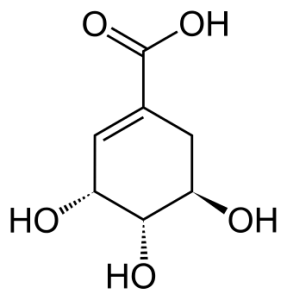
Shikimic acid is a precursor to a variety of phenolic compounds, which protect plant hormones from oxidation (Schneider & Wightman, 1974).
Tomato plant seeds that were presoaked with shikimic acid later resulted in plants that had a greater number of leaves, taller shoots and greater biomass (Al-Amri, 2013, p. 341).
The application of shikimic acid to tomato seeds led to an increased leaf area within the tomato plant and to an increased total sugar content in the tomatoes [meaning, greater nutrient density]. Also, shikimic acid-soaked tomato seeds resulted in increased nitrogen, phosphorous and potassium fractions, as well as drastically increased rates of transpiration and stomatal conductance in the tomato plant leaves (Al-Amri, 2013, pp. 342-343).
The effects of shikimic acid in terms of yield and protection against antagonists was compared to the effects of a fungicide on cowpea plant seeds and cowpea plants.
Application of shikimic acid increased the root to shoot ratio of faba beans (Aldesuquy, 2015, p. 3).
When applied to faba bean plants infected with a fungal pathogen, shikimic acid relieved the pathogen’s effects on the active and healthy growth of the fava bean shoot (Aldesuquy, 2015, p. 3).
Shikimic acid led to increased contents of nitrogen, protein and nucleic acid in faba bean plants (Aldesuquy, 2015, p. 4).
Shikimic acid caused an increase in faba bean plant root length, which led to an increase in water uptake rates and therefore the fresh weight of the plant roots and shoots (Aldesuquy, 2015, p. 5).
When applied to black eyed peas, shikimic acid increased yields in pods, pod length, number of seeds per pod, seed biomass and seed protein content (Aldesuquy & Ibrahim, 2000, p. 277).
Shikimic acid increased root length of cowpea plants throughout the growth periods (Aldesuquy & Ibrahim, 2000, pp. 281-282).
The production of photosynthetic pigments is increased in cowpea plant leaves by the application of shikimic acid (Aldesuquy & Ibrahim, 2000, p. 282). Photosynthetic activity is also “massively” increased by the application of shikimic acid (Aldesuquy & Ibrahim, 2000, pp. 286).
CINNAMIC
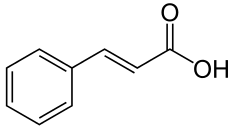
Pretreatment with cinnamic acid resulted in higher antioxidant activity in cucumber leaves and increases cucumber drought tolerance (Sun, Nie, Gao, Dai, & Bai, 2012, p. 641).
Snapdragons treated with t-cinnamic acid retained more chlorophyll (60%) than the control (Talaat, Khattab, & Ahmed, 2014, p. 360).
Cinnamic acid, when applied as a foliar spray, significantly increased the growth of shoots, the biomass and the number of branches per plant (Talebi, Hadavi, & Jaafari, 2014, p. 355).
Cinnamic acid treatments led to seed yield and oil content increases (Talaat, Khattab, & Ahmed, 2014, p. 355).
Cinnamic acid relieved salt stress on rhizobacteria (Liu, Wu, Yang, Liu, & Pan, 2010, p. 661).
FERULIC
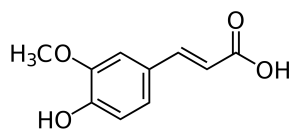
Ferulic acid is a major component of plant cell walls and also, together with dihydroferulic acid, comprises lignocellulose, which gives the cell wall rigidity (Kumar & Pruthi, 2014, p. 86).
Derivatives of ferulic acid, the most abundant hydroxycinnamic acid in plants, are important components in the plant cell wall structure, contributing to its rigidity and strength (Matthew & Abraham, 2004, p. 59).
Ferulic acid is a universal plant constituent that is found largely in seeds and leaves (Graf, 1992, p. 435). Ferulic acid is common to, amongst other plants, grasses, grains, vegetables, flowers, fruits leaves, beans, artichoke, peanuts, nuts, wood, corn husks, rice, wheat, oats and pineapple (Kumar & Pruthi, 2014, p. 87).
Ferulic acid increased soil respiration and microbial biomass in fallow or almost-fallow soils (Sparling, Ord, & Vaughan, 1981, p. 455).
BENZOIC
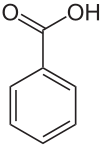
Benzoic acid, when applied as a foliar spray, significantly increased the growth of shoots, the biomass and the number of branches per plant (Talebi, Hadavi, & Jaafari, 2014, p. 355).
Foliar acid applications of benzoic acid also increased the chlorophyll, carotenoid, soluble and insoluble carbohydrate, gibberellin (plant hormone that promotes stem elongation, germination and flowering), indole-3-acetic acid and cytokinin (plant hormone that promotes tissue growth and budding) (Talebi, Hadavi, & Jaafari, 2014, pp. 359-362).
Benzoic acid applications increased fruit yield by almost fifty percent, and benzoic acid-treated fruits showed higher values in total dissolved solids than untreated fruits (Benavides-Mendoza & Ramirez, 2012, p. 251). Also, benzoic acid treated plant seeds showed greater oil yields, which could be from greater vegetative growth and greater nutrient uptake (Talaat, Khattab, & Ahmed, 2014, p. 362).
Benzoic acid is produced by plants and secreted towards the plants’ root systems, where it can help the plant assimilate nutrients from the growing medium (Benavides-Mendoza & Ramirez, 2012, p. 251).
Benzoic acid may act like a stress-signaler in plants, contributing to an increased tolerance to soil salinity. In addition, benzoic acid may increase hardiness (in cell walls), antioxidant synthesis and mitochondrial activity (cellular respiration) (Benavides-Mendoza & Ramirez, 2012, pp. 251-253).
Benzoic acids impart protection for the plant within the plant rhizosphere (Chessin & Zipf, 1990).
PROTOCATECHUIC
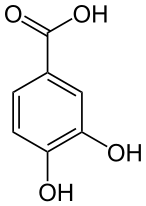
Protocatechuic acid has been documented as naturally occurring in plums, gooseberries, grapes, almonds, carrots, mushrooms, star anise, melissa, rosemary, Chinese cinnamon, Hibiscus sabdariffa L., Japanese ginkgo, St. John’s wort and also in the plant-based products, olive oil and white wine (Kakkar & Bais, 2014, pp. 1-2).
Protocatechuic acid is structurally similar to gallic and caffeic acids, well known antioxidants (Kakkar & Bais, 2014, p. 2).
ACETIC
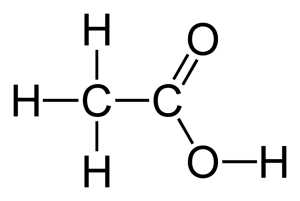
Acetic acid stimulates chloroplast development and also stimulates the entire photosynthetic system of a plant (Aldesuquy & Ibrahim, 2000, p. 284).
Acetic acid and its derivatives are found in a wide variety of concentrations in a number of types of plant tissues (Bandurski & Schulze, 1977, p. 212).
A form of acetic acid, called indole-3-acetic acid is the most common plant hormone associated with the elongation of plant cells, and it controls many primary functions in the plant (Leveau & Lindow, 2005, p. 2365).
Plant-growth promoting rhizobacteria (root-colonizing bacteria that coexist with plants in symbiotic and/or mutualistic relationships), such as Pseudomonas putida–which enhances plant growth (Glick, Ghosh, Liu, & Dumbroff, 1997, p. 253)–proliferate when exposed to acetic acid (Leveau & Lindow, 2005, p. 2369). When inoculated into the rhizosphere of plants, Pseudomonas putida, which produces indole-3-acetic acid,, contributes to a two to threefold increase in plant root length (Caron, Patten, & Ghosh, 1995; Glick, Brooks, & Pasternak, 1986).
Higher (vascular) and lower plants have the capacity to produce indole-3-acetic acid; even bacteria, fungi and algae can produce indole-3-acetic acid that plants can use and that could have a marked impact on plant proliferation (Shahab, Ahmed, & Kahn, 2009, p. 1312).
Scientists have discovered evidence that plant-associated bacteria produce indole-3-acetic acid, which is produced by plants as well, and which is key for plant growth and development (Shahab, Ahmed, & Kahn, 2009, p. 1313). Rhizobacterial-produced indole-3-acetic acid is possibly responsible for longer plant roots with increased root hairs and root laterals, which increases nutrient uptake in the plant (Shahab, Ahmed, & Kahn, 2009, p. 1314).
The main auxin in plants, indole-3-acetic acid controls many primary physiological activities in the plant, including elongation of cells (Shahab, Ahmed, & Kahn, 2009, p. 1313).
Acetic acid has significant antibacterial effects in the rhizosphere of plants within different types of soils (Park et al., 2011).
MALIC
Arabidopsis thaliana, a mustard green in the Brassicaceae family, secretes malic acid signals and recruits a beneficial rhizobacterium called Bacillus subtilis (Rudrappa, Czymmek, Pare, Bais, & American Society of Plant Biologists, 2008, p. 1547).
Malic acid, when applied to “treasure flower” plants, increased plant biomass (plant dry weight and root fresh weight) significantly, as well as plant height and the length of plant peduncles (fruit or flower-bearing stalks). Additionally, malic acid-treated plants bore longer-lasting flowers (Talebi, Hadavi, & Jaafari, 2014, p. 1).
Malic acid may have helped strengthen the root systems of the “treasure flower” plants to which it was applied, which is what lead to stronger shoot systems in the plant (Talebi, Hadavi, & Jaafari, 2014, p. 4).
Malic acid contributed to a powdery mildew tolerance increase and increases in plant height, leaf count, shoot and root fresh and dry weights of the dill plant (Jafari & Hadavi, 2011).
Malic acid demonstrated significant antibacterial effects in different types of soil and rhizospheres (Park et al., 2011, p. M293).
In a study of tomato, cucumber and sweet pepper plants, plant roots were discovered to exude a greater amount of organic acids than sugars, a major portion of which was malic acid (Doornbos, van Loon, & Bakker, 2012, p. 229).
PHENYLACETIC
The plant auxin phenylacetic acid is present at physiologically active concentrations in the shoots of a variety of crop plants, such as tomatoes, tobacco, sunflowers, peas, barley and maize (Wightman & Lighty, 1982). It is abundant in vascular (higher) and also in non-vascular (lower) plants, such as seaweeds (Korasick, Enders, & Strader, 2013, p. 2543).
Phenylacetic acid significantly enhanced the elongation of the internodal segments in the common bean plant (Phaseolus vulgaris) (Small & Morris, 1990, p. 329).
Phenylacetic acid applications enhanced the formation of Arabidopsis thaliana lateral roots (Sugawara et al., 2015, p. 1642).
In Arabidopsis thaliana, PAA levels are higher in the seeds and complete flower heads than in the stems and ground leaves (Sugawara et al., 2015, p. 1650).
Phenylacetic acid may also play “an important role as an auxin in many aspects of plant growth and development” (Sugawara et al., 2015, p. 1650).
Phenylacetic acid was used to promote multiple shoot formation and primary shoot elongation (Giridhar, Dirisala, Bandapalli, Rajasekaran, & Gokare, 2003, p. 463)
Phenylacetic acid promoted tomato plant growth (Pearse, 1936). ARTICLE en route.
SUCCINIC
Succinic acid, when used to treat plant roots, increased plant productivity and growth, and it also contributed to the increase in root formation when applied to roots, stems and leaves (Kinnersley, U.S. Patent No. US 5604177 A, 1997).
Succinic acid decreased the growth and germination of a pathogenic soil borne-fungus (Wu et al., 2011, p. 404).
In a study of tomato, cucumber and sweet pepper plants, plant roots were discovered to exude a greater amount of organic acids than sugars, a major portion of which was succinic acid (Doornbos, van Loon, & Bakker, 2012, p. 229).
Phosphate-solubilizing bacteria that increase P-uptake by plants, a process that is helpful to plant growth, were reported to produce aliphatic organic acids, including succinic acid (Chen et al., 2006, p. 33).
Succinic acid contributed to a root mass increase when applied to seedling roots, possibly explaining that the plant-growth promoting rhizobacteria, Pseudomonas putida, produces this and other organic acids in the rhizosphere and thus enhances plant growth (Yoshikawa, Hirai, Wakabayashi, Sugizaki, & Iwamura, 1993, p. 1150).
LACTIC
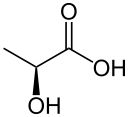
Lactic acid is found in many plants, one of which is the raspberry (Kretovich, 2013, p. 137). Large amounts of lactic acid accumulate in barley and many other plants (U.S. EPA Office of Pesticide Programs Biopesticides and Pollution Prevention Division, 2009).
Bacteria that produce lactic acid (“lactic acid bacteria”) improved seed germination quality and increased seedling strength and health when plant seeds were treated with said plant growth-promoting rhizobacteria. Additionally, the incidence of disease in same seedlings was reduced by sixty-three percent as a result of seed treatment (Murthy, Malini, Savitha, & Srinivas, 2012, p. 60).
Lactic acid has been used as a plant growth regulator since at least 1988, when an exemption from the tolerance level requirement law was made for it (U.S. EPA Office of Pesticide Programs Biopesticides and Pollution Prevention Division, 2009, p. 5).
Lactic acid, a plant-growth promoting rhizobacteria produced by lactobacilli, is a wide-spectrum antibiotic that helps defend the plant (Beneduzi, Ambrosini, & Passaglia, 2012, p. 2). Lactic acid demonstrated significant antibacterial effects in different types of soil and rhizospheres (Park et al., 2011, p. M293).
Increased duckweed and corn plant biomass (dry weight more than doubled), chlorophyll accumulation and root growth, which may be due to an increase in the plant’s ability to assimilate nutrients, resulted from being grown in a medium saturated with a polymer of lactic acid (Kinnersley, Scott, III, Yopp, & Whitten, 1990, p. 137).
Root mass increased by forty percent when plants were treated with a half and half mixture of lactic acid and succinic acid (Yoshikawa, Hirai, Wakabayashi, Sugizaki, & Iwamura, 1993, p. 1150).
Plants treated with condensation products of lactic acid show a decreased amount of nutrients needed for growth and better resistance to toxicity caused by excess salt (Kinnersley, U.S. Patent No. US RE35320 E, 1996).
An isomer of lactic acid can stimulate growth of all plants and plant parts, including fruit and fruit-bearing plants (Young, U.S. Patent No. US 4863506 a, 1989).
Both a compound from and a form of lactic acid increased leaf size, number of pods, number of beans and bean and plant biomass (Chang, Mueller, & Iannotti, 1996, p. 223).
Lactic acid is one of the organic acids secreted during phosphate solubilization by plant-growth promoting phosphate solubilizing bacteria, which increasing plant yields when inoculated into the rhizosphere (Chen et al., 2006, p. 33).
